Acid-Fast Stain- Principle, Procedure, Interpretation and Examples
It is the differential staining techniques which was first developed by Ziehl and later on modified by Neelsen. So this method is also called Ziehl-Neelsen staining techniques. Neelsen in 1883 used Ziehl’s carbol-fuchsin and heat then decolorized with an acid alcohol, and counter stained with methylene blue. Thus Ziehl-Neelsen staining techniques was developed.
The main aim of this staining is to differentiate bacteria into acid fast group and non-acid fast groups.
This method is used for those microorganisms which are not staining by simple or Gram staining method, particularly the member of genus Mycobacterium, are resistant and can only be visualized by acid-fast staining.
Principle of Acid-Fast Stain
When the smear is stained with carbol fuchsin, it solubilizes the lipoidal material present in the Mycobacterial cell wall but by the application of heat, carbol fuchsin further penetrates through lipoidal wall and enters into cytoplasm. Then after all cell appears red. Then the smear is decolorized with decolorizing agent (3% HCL in 95% alcohol) but the acid fast cells are resistant due to the presence of large amount of lipoidal material in their cell wall which prevents the penetration of decolorizing solution. The non-acid fast organism lack the lipoidal material in their cell wall due to which they are easily decolorized, leaving the cells colorless. Then the smear is stained with counterstain, methylene blue. Only decolorized cells absorb the counter stain and take its color and appears blue while acid-fast cells retain the red color.
Summary of Acid-Fast Stain
| Application of | Reagent | Cell color |
| Acid fast | Non-acid fast |
| Primary dye | Carbol fuchsin | Red | Red |
| Decolorizer | Acid alcohol | Red | Colorless |
| Counter stain | Methylene blue | Red | Blue |
Procedure of Acid-Fast Stain
- Prepare bacterial smear on clean and grease free slide, using sterile technique.
- Allow smear to air dry and then heat fix.
Alcohol-fixation: This is recommended when the smear has not been prepared from sodium hypochlorite (bleach) treated sputum and will not be stained immediately. M. tuberculosis is killed by bleach and during the staining process. Heat-fixation of untreated sputum will not kill M. tuberculosis whereas alcohol-fixation is bactericidal.
- Cover the smear with carbol fuchsin stain.
- Heat the stain until vapour just begins to rise (i.e. about 60 �C). Do not overheat. Allow the heated stain to remain on the slide for 5 minutes.
Heating the stain: Great care must be taken when heating the carbol fuchsin especially if staining is carried out over a tray or other container in which highly fiammable chemicals have collected from previous staining. Only a small fiame should be applied under the slides using an ignited swab previously dampened with a few drops of acid alcohol or 70% v/v ethanol or methanol. Do not use a large ethanol soaked swab because this is a fire risk.
- Wash off the stain with clean water.
Note: When the tap water is not clean, wash the smear with filtered water or clean boiled rainwater.
- Cover the smear with 3% v/v acid alcohol for 5 minutes or until the smear is sufficiently decolorized, i.e. pale pink.
Caution: Acid alcohol is fiammable, therefore use it with care well away from an open fiame.
- Wash well with clean water.
- Cover the smear with malachite green stain for 1–2 minutes, using the longer time when the smear is thin.
- Wash off the stain with clean water.
- Wipe the back of the slide clean, and place it in a draining rack for the smear to air-dry (do not blot dry).
- Examine the smear microscopically, using the 100 X oil immersion objective.
Interpretation of Acid-Fast Stain

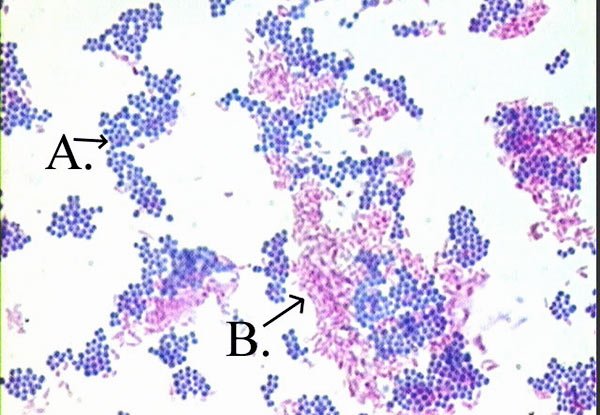
Acid fast: Bright red to intensive purple (B) , Red, straight or slightly
curved rods, occurring singly or in small groups, may appear beaded
Non-acid fast: Blue color (A)
Examples of Acid-Fast Stain
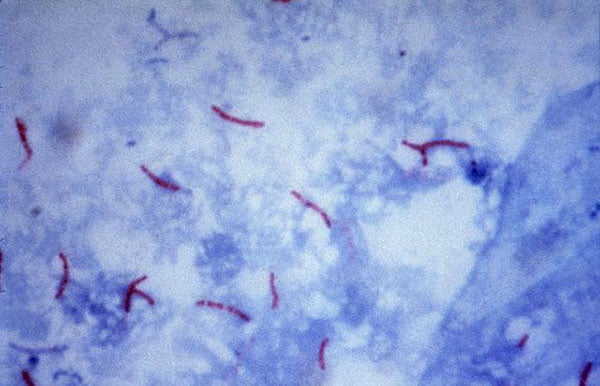
Acid-fast: Mycobacterium tuberculosis, Mycobacterium smegmatis.
Non-Mycobacterial bacteria: Nocardia
Coccidian Parasites: Cryptosporidium
Similar Posts:
- Endospore Staining- Principle, Reagents, Procedure and Result
- Capsule Staining- Principle, Reagents, Procedure and Result
- Gram Staining: Principle, Procedure, Interpretation, Examples and Animation
- Negative Staining- Principle, Reagents, Procedure and Result




